Carbon Sequestration in a Mass of RCA
The hydrated cement of concrete binds carbon dioxide from the atmosphere. This reaction is called carbonation and it permanently stores carbon in concrete. Carbon dioxide penetrates the concrete from the surface and the phenomenon slows down as a function of time, until practically stops in about 40 years. During its service life, a concrete apartment building absorbs 10–15% of the carbon dioxide released in calcination. When a concrete structure comes to the end of its service life, it is most often demolished and crushed. Here, more than 1000-fold of “fresh” concrete surface is exposed, which can react with atmospheric carbon dioxide. Carbonation is a condition sensitive phenomenon and in practice the most relevant parameters are air contact (=carbon dioxide) and relative humidity inside concrete (correlates with relative humidity of air). The carbon sequestration of concrete has been studied to certain extent and the recycling phase has been identified as very relevant for the reasons mentioned. However, research on the conditions affecting carbonation inside a mass of crushed concrete is relatively light, although carbonation itself is well known.
(https://concretesolution.fi/tutkimustuloksia-maailmalta-betoni-on-hiilinielu/)
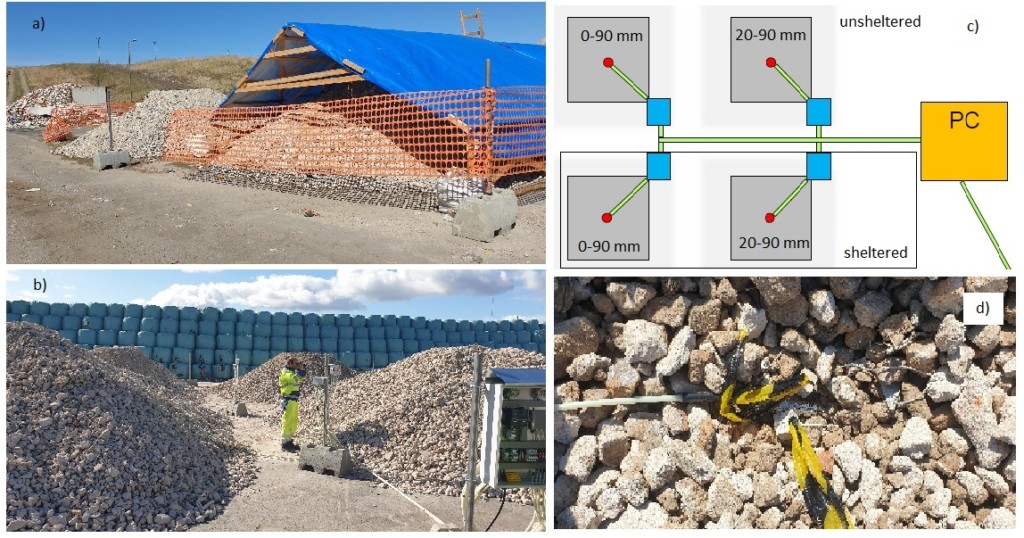
The conditions essential for carbon sequestration (carbon dioxide content, humidity) inside the mass of crushed concrete have been unknown. This is essential information when optimizing the storage and use of recycled concrete also as a carbon sink. A research environment was set up at the Topinoja recycling center in Turku to investigate these conditions and how these conditions could be easily improved to enhance the carbon sink of recycled concrete.
Four different piles were measured for carbon dioxide content and humidity. The measuring points in each pile were at four different depths. The crushed stone used was 0–90 mm as well as 20–90 mm and two of the piles were sheltered from direct rain. In the two-meter-high piles, the measuring points were at a distance of 0.25 m, 0.5 m, 1 m and 2 m from the upper surface of the pile. The measurements took place between 9/2021–11/2022.
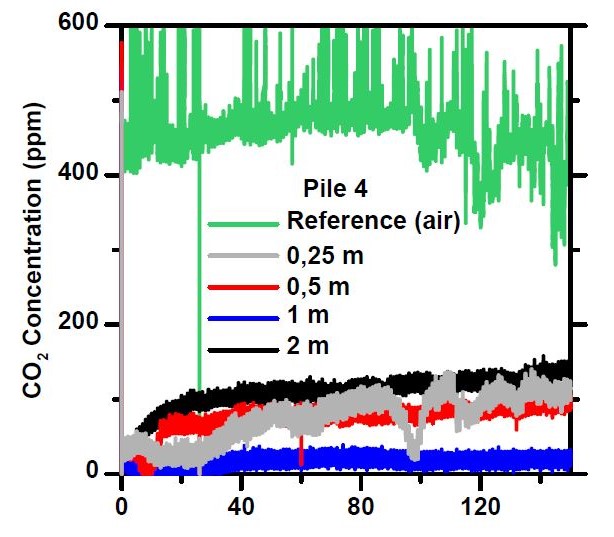
Depending on the depth of measurement, the internal carbon dioxide concentration of a conventional 0–90 mm crushed concrete pile was in the range of 20–130 ppm, i.e., well below the reference concentration measured in the air, as expected. At depths of 0.25 m-1 m, the CO₂ concentration remained close to 100 ppm. Also, there was clearly carbon dioxide measured at the very bottom of the pile, though in lower concentrations. Thus, carbon dioxide, which is essential for carbonation, is also found deep in the crushed mass, although the low concentration slows down the reaction and the concentration of 20 ppm corresponds to a carbonatization rate of about 20 % compared to direct air contact.
In a pile of sifted recycled concrete (20–90 mm), the mass is clearly ventilated and the CO₂ concentrations measured at all depths are close that of the reference concentration. Once the finer material has been screened out, practically all particles are in air contact and thus under optimal conditions for carbon sequestration.
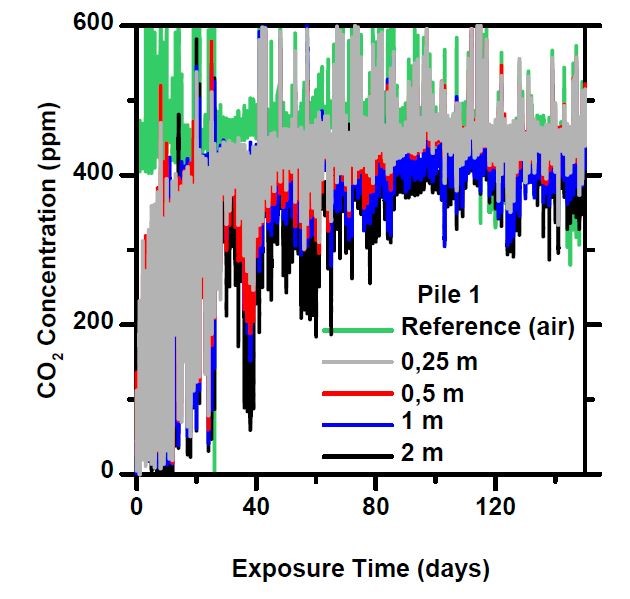
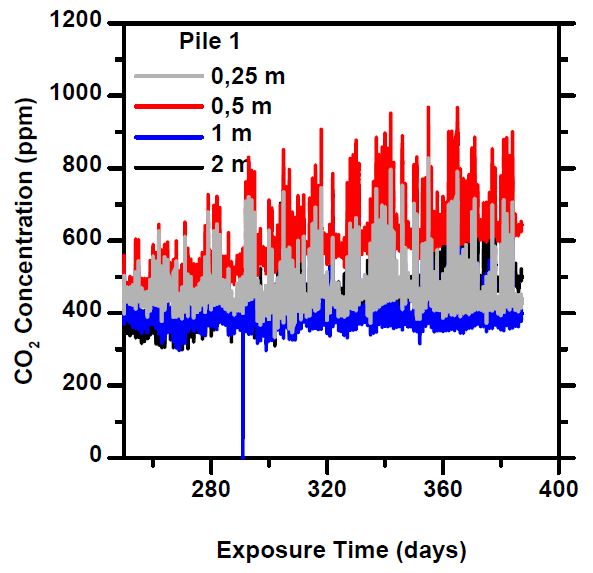
Especially from the 20-90 mm piles, the “period of intense absorption” at the beginning of the measurement is notable, in which freshly crushed concrete reacts eagerly, sequestering atmospheric carbon dioxide, causing the concentration to temporarily drop to close to zero. When a new mass of air enters the pile, the CO₂ concentration rises again for a while, until it is bound to the concrete. This phase lasted about a month, after which the concentrations stabilized to a constant level.
The humidity measurement sensors showed 100% relative humidity practically throughout the study, which would have indicated a complete water saturation between the particles. This was not a probable status and the disassembly of the piles provided visual evidence that the screened crushed concrete piles were dry inside, as might be expected. However, the moisture conditions of the mass containing the fine material requires further study, as this is a relevant parameter affecting carbonation.
Known concrete samples were placed at all measuring points, from which the content of calcium carbonate was measured. The exposure time to these samples was quite short and there were only a few samples, but the data obtained supports the result of the condition measurements. The reference samples from the sifted piles had absorbed more carbon dioxide than the heaps containing finer matter. Rain protection appeared to have less impact on this measurement, which in part also suggests that the humidity inside the pile is lower than in direct rain contact (as well as the measured 100% humidity being an error measurement).
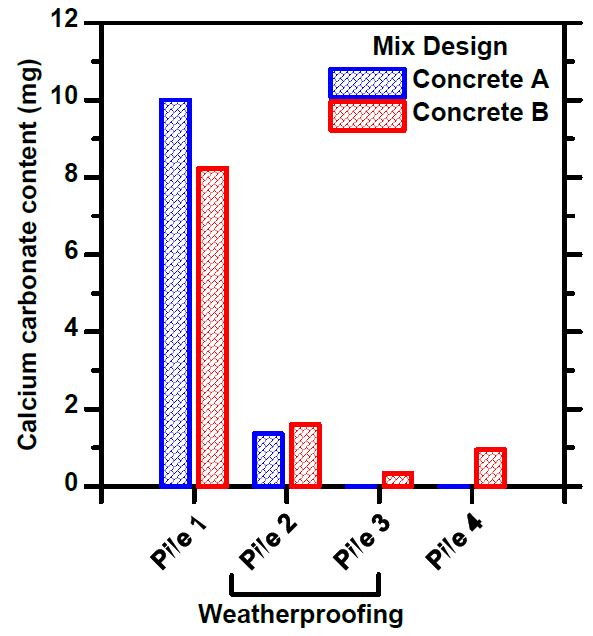
By manipulating the particle distribution of crushed concrete in a large mass, it is possible to influence the internal conditions of the pile and thus promote carbonation. Even in conventional crushed concrete, where the entire particle distribution is present, carbon dioxide is clearly present even in the deeper layers of the mass, although carbonation is slower. The sifted mass dries quickly, so sheltering from direct rain may be unprofitable in relation to the cost. The humidity conditions of ordinary crushed concrete remain a question. Based on the results obtained, it is a good idea to start calculations on how crushed concrete could be used in applications and stored and how it would affect the carbon sequestration of recycled concrete.